Which of the Following Chemical Equations Describes a Dehydration Reaction
Disaccharide H2O monosaccharide monosaccharide D. A substance that reacts with water.
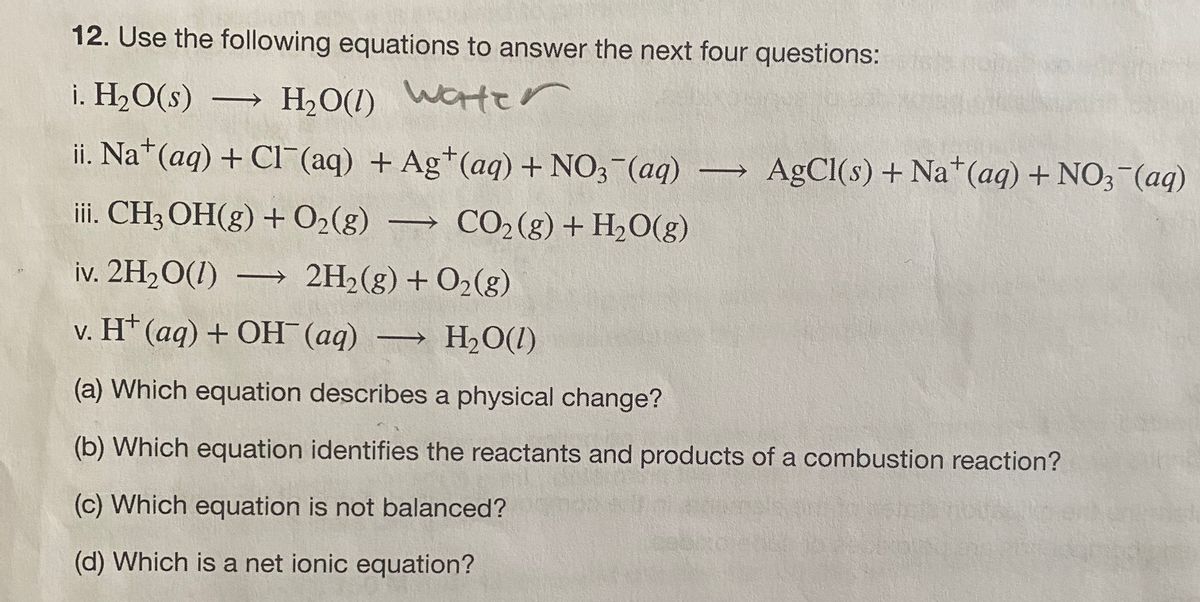
Answered 12 Use The Following Equations To Bartleby
For example acetic acid CH 3 COOH forms acetic anhydride CH 3 CO 2 O and water by the dehydration reaction.

. If CO2 is bubbled into a beaker containing pure freshly distilled water which of the following graphs correctly describes the results. Disaccharide H2O monosaccharide monosaccharide. The ozonolysis product of A and B are asked Sep.
Methyl bromide 2 C H 3 B r 2 N a dry ether ethane C H 3 C H 3 2 N a B r b Finkelstein reaction. Monosaccharide monosaccharide disaccharide H2O. Which of the following chemical equations describes a dehydration reaction.
Dehydration synthesis condensation reaction between sugar molecules. The classic example of a dehydration reaction is the Fischer esterification which involves treating a carboxylic acid with an alcohol to give an ester. Disaccharide monosaccharide monosaccharide H2O.
3 Which of the following chemical equations describes a dehydration reaction. Autocatalytic Equation Describing The Change In Molecular Weight During Hydrolytic Degradation Of Aliphatic Polyesters Biomacromolecules. A dehydration reaction is a chemical reaction in which a reactant loses a water molecule.
The Wurtz reaction named after Charles Adolphe Wurtz is a coupling reaction whereby two alkyl halides are reacted with sodium metal in dry ether solution to form a higher alkaneGeneral reactionRX2NaXR DryEther AlkaneRR 2NaXEx2CH 3 CH 2 Br DryEtherNa CH 3 nbutaneCH 2 CH 2 CH 3. In a hydrolysis reaction the water molecule act as a chemical agent that breaks the chemical bond of a large molecule to form smaller molecules. Carbon dioxide CO2 is readily soluble in water according to the equation CO2 H2O----- H2CO3.
A disaccharide monosaccharide monosaccharide H2O B disaccharide H2O monosaccharide monosaccharide. Dehydration of 22344-pentamethy 1-3-pentanol gave two alkenes A and B. Dissacharide is a structure which consists of two molecules of sugar undergoes breakdown by the hydrolysis reaction.
Monosaccharides disaccharides and polysaccharides. Chemical Describes Hydrolysis the. One way to accomplish this goal would be to.
Disaccharide monosaccharide monosaccharide H2O о O C. Often such reactions require the presence of a dehydrating agent ie. Disaccharide is a bigger molecule as compared to a monosaccharide.
Dehydration reactions are also involved in the production of many polymers. Monosaccharide monosaccharide H20 disaccharide O B. Alkyl halides react with sodium dry ether to form higher alkanes.
Which one of the following chemical equations describes a hydrolysis reaction. Evaluation Of The Progress Of Protein Hydrolysis Navarrete Del Toro 2003 Current Protocols In Food Analytical Chemistry Wiley Online Library. Monosaccharide monosaccharide disaccharide H2O.
The reaction between alkyl chlorides bromides and NaI dry. Which of the following chemical equations describes a dehydration reaction. Describe its role using words and chemical equations.
What role does phosphoric acid play in this reaction. A disaccharide monosaccharide monosaccharide H2O B disaccharide H2O monosaccharide monosaccharide. D disaccharide H2O monosaccharide monosaccharide.
Formation of maltose from glucose monomers. What is a common chemical. Dehydration synthesis involves the formation of new chemical bonds between two molecules which leads to the formation of new compounds.
Monosaccharide monosaccharide disaccharide H2O disaccharide H2O. A reaction occurs with the loss of water molecule at each step. The loss of water molecule can occur due to reaction between two functional groups like OH -NH 2 or COOH.
D disaccharide H2O monosaccharide monosaccharide. A dehydration reaction or condensation reaction is the process in which. Or in other world disaccharide is twice the size of monosaccharide.
View the full answer. Monosaccharide monosaccharide H2O disaccharide. Predict the dehydration products for the following alcohols.
The four main categories of large biological molecules present in living. RCO 2 H ROH RCO 2 R H 2 O. C disaccharide monosaccharide monosaccharide H2O.
Water molecules are produced as a polymer is formed from monomers Monomers are joined together in a reaction in which two molecules are covalently bonded to each other through the loss of a water molecule. Which of the following chemical equations describes a dehydration reaction. Reactions that produce acid anhydrides are dehydration reactions.
Explore the definition and examples of a dehydration reaction and discover the difference between. Testosterone and estradiol are male and female sex hormones respectively in many vertebrates. Carbonic acid H2CO3 is a weak acid.
2 CH 3 COOH CH 3 CO 2 O H 2 O. Describe its role using words and chemical equations. Why does cyclohexene distill over to the receiving flask while cyclohexanol remains in the reaction flask.
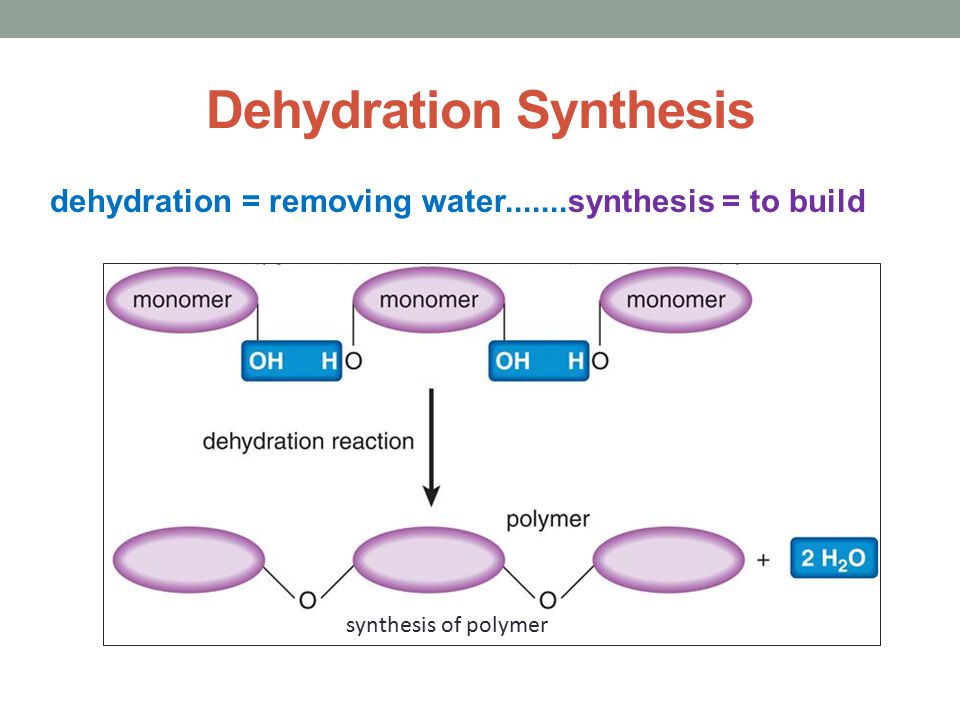
What Do The Following Equations Represent Ppt Download
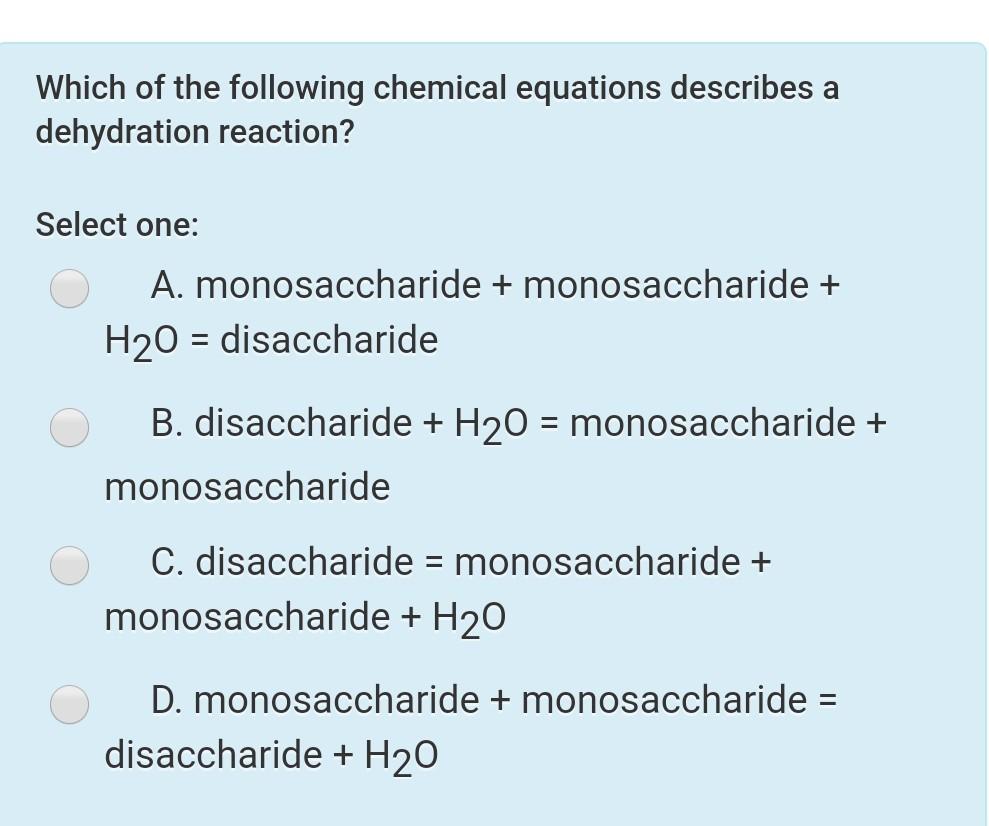
Solved Which Of The Following Chemical Equations Describes A Chegg Com

Solved Which Of The Following Chemical Equations Describes A Chegg Com
Comments
Post a Comment